Periodic Classification of Elements
1. Introduction
Till date, humans have come across 118 different elements. Out of the 118, only 94 are found in nature. So some scientists decided to classify these elements. Many scientists came up with many different methods, but not all of them were great. Let's look at some of them.
1.1 Some terms to keep in mind
- Atomic number: The number of protons* present in an atom.
- Atomic mass: Sum of the number of protons and neutrons in an atom.
- Valence electrons: Electrons in the outermost orbit.
- Valency: Number of electrons given, taken or shared to make a bond.
*Number of electrons can be considered in a neutral atom. But generally, not many neutral atoms roam around freely in nature.
2. Early attempts at classification of elements
2.1 Dobereiner's Triads
Johann Wolfgang Dobereiner in 1817, arranged elements into groups of 3 which he called 'triads'. The idea of these 'triads' was that the arithmetic mean of the atomic masses of the first and the third elements is the atomic mass of the second element. The triads were arranged in the increasing order of atomic masses.
For example, consider the atomic masses of the following:
Lithium (Li) = 6.9 u
Sodium (Na) = 23 u
Potassium (K) = 39 u
Li + K = 45.9 ( approx. = 46 )
46/2 = 23, which is the atomic mass of Na
Dobereiner could only find three triads in nature, therefore his idea of triads was dismissed.
| Triad 1 | Triad 2 | Triad 3 |
|---|---|---|
| Lithium (6.9 u) | Calcium (40 u) | Chlorine (35.5 u) |
| Sodium (23 u) | Strontium (88 u) | Bromine (80 u) |
| Potassium (39 u) | Barium (137 u) | Iodine (127 u) |
2.2 Newland's law of octaves
After Dobereiner, John Newland tried to classify the elements. Just like Dobereiner, he used atomic mass to classify elements.
In his time only 56 elements were discovered. He arranged them starting with hydrogen and ending with thorium. He found that when he arranged elements in that order, every eighth element exhibited similar properties. He compared this pattern to octaves in music.
This is what he came up with:
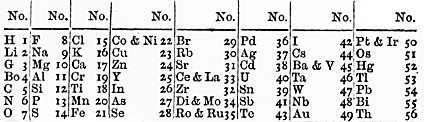
Why Newland's Law of Octaves didn't work:
- The patterns mentioned above occurred only till Calcium
- His table was applicable only to the 56 elements that were known at that time, and it didn't occur to Newland that new elements would ever be found.
- He even tried to fit two into one slot and left some slots blank, and had many such inconsistencies that ended up ruining the beauty of the table.
All these reasons made his table irrelevant
2.3 Mendeléev's Periodic Table
By the time Mendeléev started his work on the periodic table, there were 63 known elements.
Just like the other two, he used atomic mass as the base of his work.
He studied elements and arranged them on the basis of their chemical properties, including their hydrides and their oxides. While doing this, he noticed that there was a periodic recurrence of some properties.
On this basis, he came up with this Periodic Law:
“Properties of elements are a periodic function of their atomic masses."
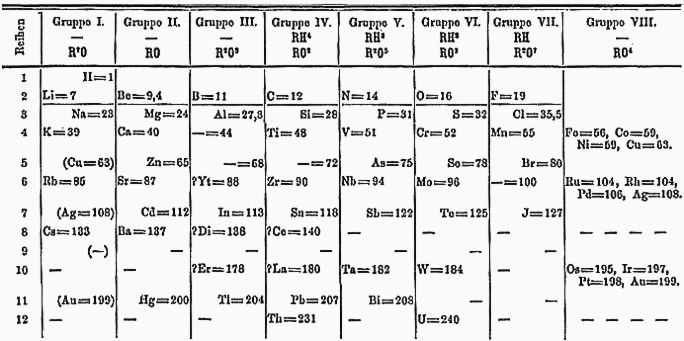
The 'R' above each group denotes his idea that any element from that group can replace that 'R' to make a molecule. He called the horizontal rows periods and the vertical columns groups.
2.3.1 Advantages of Mendeléev's Periodic Table
He was so sure of his table's accuracy that he even left some gaps in his periodic table. He knew that more elements will be discovered in the future to fill those gaps. He named these elements with the eka- prefix (Sanskrit word for "one") and the element they shared a lot of properties with.
Eka-aluminium → Gallium
Eka-boron → Scandium
Eka-silicon→ Germanium
Another advantage of Mendeléev's periodic table was that when the noble gases were found, they could be placed in a separate group without disturbing the existing order.
2.3.2 Limitations of Mendeléev's Periodic Table
-
Mendeléev failed to give a proper position for Hydrogen, as it exhibited both acidic and alkaline, metallic and non-metallic properties. This is why even today some periodic tables separate Hydrogen from the rest of the elements.
-
When isotopes were discovered, Mendeléev's periodic table was not able to properly position them either.
-
It became difficult to predict how many elements could be discovered between two elements once the atoms started getting heavier as atomic masses do not increase in a regular manner.
2.4 Moseley's Modern Periodic Table
In 1913 Henry Moseley decided to arrange the elements according to atomic number. He also took Mendeléev's periodic law and modified it such that it reads:
“Properties of elements are a periodic function of their atomic number."
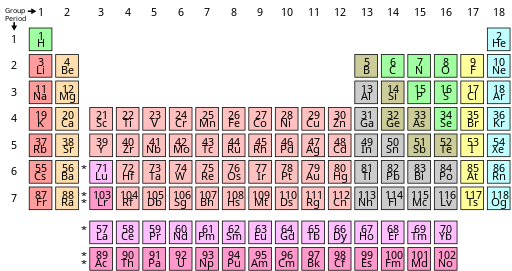
2.4.1 Position of elements
- In the modern periodic table there are 18 groups and 7 periods.
- All the elements in a group have the same number of valence electrons.
- Number of shells increases as we go down a group. E.g. Elements of Group 17 (aka halogens), including Fluorine, Chlorine, Bromine, Iodine, all have 1 electron in their outermost shell.
- Number of valence electrons increases as we go across a period.
2.4.2 Trends in the Modern Periodic Table
Valency
- Across a period, valency first increases then decreases.
- In a group, valency remains constant.
Atomic size
-
Atomic size refers to the radius of an atom.
-
Across a period, atomic size decreases because more electrons get added and the electrons get pulled towards the nucleus.
-
In a group, atomic size increases because new shells get added and thus increasing the radius.
Metallic and Nonmetallic properties
-
When we observe the periodic table, we can see that many metals can be found on the left hand side of the periodic table, and many nonmetals are on the right.
-
Separating the two, there's a zig-zag line of metalloids. They are just some elements showing both metallic and nonmetallic characters. E.g. Silicon, Boron, Germanium etc.
-
Metallic characteristics decrease across a period as the electrons get pulled closer by the nucleus, thus the atoms lose the tendency to give electrons. Not losing electrons is a tendency exhibited by non-metals.
-
Metallic characteristics increase down a group because more shells get added when we go down a group. More shells cause electrons to be farther from the nucleus, which increases the tendency of them being lost. Losing electrons is a tendency exhibited by metals.